OK this post was a warm up for today.
Microtubules are long hollow polymers. They are also polar. Their minus ends are inert and are found towards the cell center while their plus ends grow and shrink and are found towards the cell periphery.
Question: Why are microtubules hollow?
Well you might think that a tube provides more strength and is less flexible. Now that might be true for a cluster of microtubules bunched up together, but beyond a couple of micrometers, single microtubules are as limp as a wet noodle.
Could there be another role?
Others have claimed that various micro "cargo" could be transported inside the microtubule (also called the microtubule lumen) - similar to subway tunnels. This would have to be an active process as diffusion within the tube would be fairly slow, as specially for any molecule that had any affinity foe the inner walls of the polymer.
Perhaps microtubules store factors? Is there anything in the microtubule lumen?
As discussed in ScienceSampler, a few recent papers have surfaced that report inner-microtubule densities. These densities were seen in axonemal microtubules.
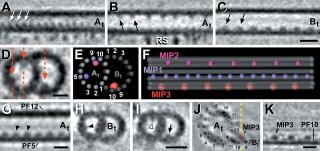
OK some more background. Axonemes are a superstructure formed by nine microtubule doublets (+ two central microtubules) and are found in long cellular projections such as flagella or cilia (like the primary cilium). To generate force a microtubule motor (dynein) slides the doublets against one another. This tends to "curve" the axoneme. Curiously this arrangement of microtubules is also seen in centrioles ... in fact a centriole like body, called the "basal body", is associated with the base (or minus end) of axonemes. Well ... okay it is not exactly the same - centrioles and basal bodies have microtubule triplets! (and no inner microtubule pair.) Despite these minor differences, it is thought that basal bodies act as a template for the axonemal microtubules.

OK lets get back to the microtubule lumen ... what about these inner microtubule densities?
The function of these may be to strenthen axonemal microtubles. But their presence does beg the question, how did they get there? The simple answer is that the axoneemal microtubules were formed that way. Since these densities are regularly spaced (see first image), and it now seems like microtubules are assembled by the addition of large chunks (rather than by monomer addition) to the plus end of the polymer, axonemal microtubules may be preassembled by the addition of specific tubulin oligomers, each oligomer having a defined lumenal appendage.
Some further notes on microtubule lumens. Tubulin subunits within microtubules accumulate modifications. One such modification (tubulin acetylation) occurs on a lysine residue that is facing the microtubule lumen. Taxol, which stabilizes microtubules, (including pre-polymerized microtubules) also binds to the inner-wall along the entire length of the polymer. So how does the tubulin acetylase and taxol reach the inner sanctum? Certain individuals have calculated how long it would take for taxol to diffuse inside a microtubule tube, and it's way too long. In addition if the acetylase diffuse inwards you would expect that tubulin near the microtubule ends (where the tube entry is) would have more acetylation than tubulin within the microtubule core. In fact what you see is that acetylated microtubules accumulate the modification in short stretches and these acetylated stretches are found along the entire length of the tube. Weird.
 The current idea is that microtubules "breathe". You see tubulin polymerizes in a spiral to form a microtubule. In this diagram, the alpha/beta tubulin monomer would be a yellow + black pair. As you can see the spiral yellow/black is imperfect, there is a seam to the microtubule. Well many researchers have postulated that microtubules may open along this seam occasionally to let things in or out.
The current idea is that microtubules "breathe". You see tubulin polymerizes in a spiral to form a microtubule. In this diagram, the alpha/beta tubulin monomer would be a yellow + black pair. As you can see the spiral yellow/black is imperfect, there is a seam to the microtubule. Well many researchers have postulated that microtubules may open along this seam occasionally to let things in or out.
And that's today's strange microtubule entry. Tomorrow I'll tell you some more bizarre microtubule facts.
- Log in to post comments
Though you may get to this in the course of discussing microtubule strangeness, there's some cool data that I think contributes to the concept of microtubule "breathing". The Nogales lab at Berkeley have done a lot of Cryo EM and crystallography work with tubulin and different GTP/GDP analogues. They found that tubulin can form large sheets and hypothesize from the structural data that these sheets fold up to nucleate a filament. If it's true, this folding should be entirely reversible, and it fits well with the concept of breathing. They had a graphic design team put together a cool animation of this (as well as more strangeness: protofilament "peeling" during depolymerization) below. All of the MT behavior shown is extrapolated from angles between dimers in different structural experiments.
http://cryoem.berkeley.edu/animations.shtml
I saw her talk about this last year. Very cool stuff. She gives one of the best structural talks.
Awesome video.