The Cytoskeleton. Now that's what you call a misnomer. It is one of the most fascinating, yet misunderstood, macromolecular assemblies of the cell. Yes, the cytoskeleton can act as a scafold onto which the rest of the cell is drapped (so to speak), but in reality the cytoskeleton is a dynamic responsive network that can mold the cell and organize the cell's contents to maximize inner-cell differentiation. Perhaps a bettler title is the psychoskeleton, as one noted researcher once called it.
 Take the microtubule cytoskeleton. This is a highly dynamic structure that grows and shrinks constantly. (Click here for more on microtubules). Most naive biologists would argue that it just hangs out and provides rigidity to the cell. Others may argue that's main role is to serve as tracks for inner cell transport. Others would point to the mitotic spindle, which is composed of microtubules. And all that is true. But one of the most important roles of the microtubule cytoskeleton (and least appreciated) is that it regulates and thus coordinates actin polymerization.
Take the microtubule cytoskeleton. This is a highly dynamic structure that grows and shrinks constantly. (Click here for more on microtubules). Most naive biologists would argue that it just hangs out and provides rigidity to the cell. Others may argue that's main role is to serve as tracks for inner cell transport. Others would point to the mitotic spindle, which is composed of microtubules. And all that is true. But one of the most important roles of the microtubule cytoskeleton (and least appreciated) is that it regulates and thus coordinates actin polymerization.
As I've noted before, actin is believed to be responsible for most of the cell morphogenesis. Actin mesh-work pushes on membranes as it grows (see this post for movies), and actin filaments and tits partner myosin help cells contract. Microtubules coordinates the location and the timing of actin polymerization. In other words the microtubule cytoskeleton is the master conductor of actin and thus cell shape.
If you depolymerize microtubules with drugs such as nocodazole, the first thing that happens is that cells appear to go bonkers. What in fact happens is that actin contracts throughout the entire cell interior and actin polymerization occurs stocastically at points all around the cell periphery. Since cells have lost their polarity they stop dead in their tracks. You can actually inhibit the nocodazole induced cell contraction with inhibitors of myosin contraction, so it's not that contraction is caused by a loss of rigidity, but that contraction is globally activated. Likewise if you lightly treat cells with low concentrations of microtubule poisons that act to dampen microtubule dynamics, cells completely freeze. It turns out that dynamic microtubules are needed to stimulate the growth of the actin mesh-work which is responsible for pushing out membranes.
In other words microtubules coordinate actin. Without this coordination, cells can't maintain their ability to have front ends and back ends - this front/back dichotomy is referred to as "cell polarity".
So how do microtubules regulate actin?
Here is where it gets interesting. The major regulators of actin based morphogenesis are the small G-proteins, rho, rac and Cdc42. These guys act like switches for the actin cytoskeleon. They stimulate actin polymerizing proteins and actin contracting proteins and other things related to morphogenesis (they also regulate a whole bunch of processes such as cell division and vesicle traffic - sound familiar?) It turns out that if you depolymerize microtubules, rho activity goes through the roof. If you freeze the microtubules, rac is turned off. Rho inturn promotes the formation of long contractile actin filaments (and also promotes the contraction of these filaments), while Rac promotes the formation of the actin mesh-work. This ability of microtubules to affect actin is known as "cytoskeletal crosstalk".
But there is more - something really strange and interesting. Guanine-triphosphate (GTP) regulates both small G-proteins and tubulin activity. In the GTP bound state tubulin polymerizes into microtubules, and G-proteins bind to their downstream targets. 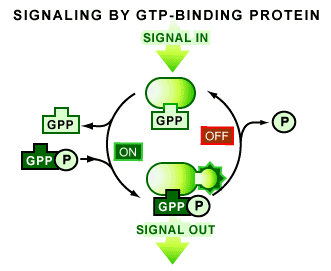 Both proteins have an intrinsic GTPase activity that breaks down the last phosphate, producing GDP and a free phosphate. GDP bound tubulin, if found at the tip of a microtubule, tends to depolymerize, GDP-bound G-proteins tend to disengage their targets. (For more on G-proteins click here). Think of G-proteins as switches. Different inputs turn them on (GTP bound state) other inputs turn them off. Microtubules are a major input for G-proteins that regulate the actin cytoskeleton. Tubulin polymer can soak up and affect the activators and inhibitors of small G-proteins, called GEFs and GAPs. GEFs (for Guanadyl Exchange Factors) help G-proteins to kick out GDP and then bind a fresh GTP molecule. GAPs (for GTPase Activating Proteins) help stimulate the G-protein's GTPase activity. (No analogues to GEFs and GAPs exist for tubulin.) Tubulin also is an input for itself but in a much more direct way - it acts as its own GAP. Tubulin is a self-referential switch. And this is why tubulin/microtubules have bizarre properties (more on that in a later post).
Both proteins have an intrinsic GTPase activity that breaks down the last phosphate, producing GDP and a free phosphate. GDP bound tubulin, if found at the tip of a microtubule, tends to depolymerize, GDP-bound G-proteins tend to disengage their targets. (For more on G-proteins click here). Think of G-proteins as switches. Different inputs turn them on (GTP bound state) other inputs turn them off. Microtubules are a major input for G-proteins that regulate the actin cytoskeleton. Tubulin polymer can soak up and affect the activators and inhibitors of small G-proteins, called GEFs and GAPs. GEFs (for Guanadyl Exchange Factors) help G-proteins to kick out GDP and then bind a fresh GTP molecule. GAPs (for GTPase Activating Proteins) help stimulate the G-protein's GTPase activity. (No analogues to GEFs and GAPs exist for tubulin.) Tubulin also is an input for itself but in a much more direct way - it acts as its own GAP. Tubulin is a self-referential switch. And this is why tubulin/microtubules have bizarre properties (more on that in a later post).
But the similarities don't end there. Both tubulin and G-proteins have similar GTP binding folds (i.e. the "Rossman fold") and although they do not share much sequence homology, both tubulin and G-proteins may have a common ancestor. This ancient GTP binding protein must be older than the emergence of eukaryotes as both tubulin and G-proteins have prokaryotic counterparts (Ftsz for tubulin, Era for small G-proteins). Now don't get me wrong, there are lots of differences, tubulin "monomer" is a dimer while G-proteins do not dimerize (as far as we know) and GTP induces different ultra structural changes in the two classes of proteins. But both "regulate" cell division, cell polarity, vesicle traffic and other cellular functions. G-proteins also influence tubulin activity ... so it's not as simple as microtubules => G-protein => actin => morphogenesis. (In fact it's probably best to write microtubules <=> G-proteins <=> actin.) Microtubules also control when and where cells attach to the substratum and this activity appears to be G-protein independent. As usual, the more you dig the more complicated it gets.
But still there is a take home message. Tubulins (and thus microtubules) are actually signalling molecules!
Ref:
Erickson HP,
Atomic structures of tubulin and FtsZ
Trends in Cell Biology (98) 8:133-137.
(More refs on request ... it's a busy day ...)
- Log in to post comments
OooOOOooo. Nice post, Alex.
I'm a bit of a psychoskeleton wannabe myself (thesis and one and a half postdocs looking at various actin/motility bits), and I reckon it's the prettiest field in cell biology ;)
That is a beautiful picture of MTs!
The cytoskeleton is a series of tubes!
**in crazy old man voice**
You are in a twisty maze of microtubules, all different
> xyzzy
Nothing happens
Joolya,
Thanks. All pics (in this post) taken by me. More MT entries are coming.