Olfaction (smell) is the most mysterious of senses, and is wrongly regarded as insignificant by most people. The sense of taste, for example, consists in large part of smell - try holding your nose next time you eat - and the recent identification of putative pheromone receptors in humans suggests that olfaction affects behaviour in as yet unknown ways.
The human nose, while not as sensitive as, say, that of a dog, can still detect very low concentrations of odorant molecules as they diffuse through the air. The initial event in the process of olfaction is the recognition of an odorant molecule by the olfactory receptors, which are proteins found in the olfactory epithelium. Olfactory receptors are transducers - they convert the 'information' in odorant molecules into electrical signals that are sent to the brain. It is only when these signals are processed in the olfactory cortex that we experience the smell.
While the higher order processing of the signals generated by olfactory receptors is relatively well understood, very little is known about how the receptors transduce the information contained in odorants into electrical signals. It has always been assumed that olfactory receptors function in the same way as other receptors - via the 'lock and key' mechanism. According to this well established model for the interaction of a receptor with its ligand (the molecule which binds to it), the receptor recognizes the three-dimensional shape of the ligand, and can only be activated by that specific molecule. Thus, in most cases, signal transduction begins with a molecular recognition event.
In the case of olfaction, however, there is a problem. A finite number of olfactory receptors recognize a seemingly infinite number of odorant molecules. So, although the shape and size of odorants is known to be important, olfactory receptors must also be detecting some other property of the odorants.
In the mid-1990s, Luca Turin, a biophysicist who was then at University College London, proposed a novel mechanism for olfactory receptor transduction. Few people, if any, know more about how the nose knows the difference between one odorant and another than Turin. He is, to borrow the title of a recent book about him, "the emperor of scent". It is because of his expertise in olfaction that the French perfume houses consulted Turin about their new fragrances.
At UCL, Turin's office doubled up as a makeshift laboratory. He spent much of his time in the long, narrow room, its walls lined from floor to ceiling with bottles of perfume, tirelessly investigating the relationship between the structures of thousands of aromatic compounds and their odours. His hypothesis was published in the journal Chemical Senses:
...olfactory receptors respond not to the shape of the molecules but to their vibrations. [The theory provides] a detailed and plausible mechanism for biological transduction of molecular vibrations: inelastic electron tunnelling.
In a non-biological system, inelastic electron tunneling is "a non-optical form of vibrational spectroscopy [which] relies on the interaction between electrons tunneling across a narrow gap between metallic electrodes and a molecule in the gap". In a biological system, such as the olfactory system, this would involve the tunneling of an electron between a suitable donor molecule and specific, electrically-charged amino acid residues within the olfactory receptor.
Turin's hypothesis was not controversial - he says it was "ignored rather than criticized". But recently, in a paper published earlier this year in Physical Review Letters, Marshall Stoneham and colleagues, of UCL's Department of Physics and Astronomy, report that they have performed calculations which suggest that Turin's theory is feasible:
We test the viability of [Turin's] mechanism using a simple but general model. Using values of key parameters in line with those of other biomolecular systems, we find the proposed mechanism is consistent both with the underlying physics and the observed features of smell, provided the receptor has certain qualities.
News of the paper has generated some interest in Turin's theory of olfaction. But Turin, of course, has always been adamant that his theory is correct. Several years ago, he set up Flexitral, a company which designs odorant molecules for use by the perfume industry. At the company's headquarters in Chantilly, Virgina, Turin and his colleagues have been using the theory to predict the smell of odorant molecules before synthesizing them. Turin's hypothesis explains not only how a limited number of olfactory receptors can detect a far larger number of odorants, but also why odorants with very similar molecular structures can smell very different, and, conversely, why molecules with different structures can have similar odours.
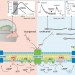
In order to gain some understanding of Turin's hypothesis, we first need to look at the structure of olfactory receptors. Olfactory receptors were first cloned by buck and Axel in 1991. In mammals, olfactory receptors are G-protein-coupled receptors (GPCRs). The GPCRs constitute the largest known protein superfamily. Mice have approximately 900 odorant receptor genes encoding 1,200 receptors, and humans have about 350 receptor genes.
GPCRs are embedded in the membrane of olfactory cells, and have a distinctive structural motif: the string of amino acids of which they are composed winds back and forth within the membrane, spanning it seven times.
GPCRs are named because they recruit intracellular proteins called G-proteins to transduce sensory signals. The exact mechanism of action of GPCRs is unknown, but very basically, it occurs as follows. When the receptor is inactive, it has an inactive G-protein bound to its intracellular surface. The binding of a ligand to the receptor's extracellular surface causes a conformational change in the receptor, which results in the G-protein being activated. The activated G-protein is released from the olfactory receptor, and then binds to, and activates, other protein molecules within the cell, initiating a chain of biochemical reactions.
According to Turin's hypothesis, olfactory receptors act like biological spectroscopes, with the transduction of olfactory stimuli depending on the detection of activity on the subatomic scale. Turin proposes that the binding of an odorant mediates inelastic electron tunneling, whereby an electron is transferred from a donor molecule to the receptor. Tunneling of electrons across the odorant's binding site activates the receptor and causes the odorant to vibrate. It is these patterns of vibrations which are specific to the odorant, and which are detected by the olfactory receptors. Even the slightest difference in molecular structure therefore produces a different vibrational spectrogram. Together, the series of receptors in the olfactory epithelium cover the vibrational spectrum, and therefore can detect all possible odorants.
So what evidence is there that electron tunnelling takes place in olfactory receptors? Turin is successfully using his theory to predict the odor of chemicals before they are synthesized. Turin's also theory makes a number of predictions about the functional properties of olfactory receptors. Firstly, because most odorants cannot undergo reduction-oxidation (or electron exchanging) reactions, the receptors must obtain the electrons used for tunnelling from another source, perhaps a soluble electron carrier or an enzyme. And, because many enzymes which transfer electrons require binding of metal ions, olfactory receptors may also be expected to have metal binding sites.
Analysis of DNA sequences of olfactory receptors shows that these predictions are correct. The olfactory receptors which have been sequenced are now known to contain a binding site for a molecule called nicotanamide adenine dinucleotide phosphate (NAD(P)H), a cofactor molecule which binds to enzymes and exchanges electrons with them. Sequence analysis also shows that olfactory receptors have sequences that are closely related to, and that function as, zinc binding sites. Zinc is known to be involved in olfacation, as a deficiency of the metal results in temporary, reversible anosmia (the inability to smell), but its exact role is unclear. Turin suggests that the zinc binding sites in the olfactory receptors are involved in binding G-proteins, and that the zinc ions themselves contribute to a molecular 'bridge' through which electrons tunnel during the transduction process.
References:
Turin, L. (1996). A spectroscopic mechanism for primary olfactory reception. Chem. Senses 21: 773-791. [Full text]
Brookes, C. et al. (2007). Could humans detect odors by phonon assisted tunneling? Phys. Rev. Lett. 98: 038101. [Abstract]
- Log in to post comments
Really interesting idea, heard about it a few years ago. Problem I have with it is this: could someone please explain to me how the neurons in which these receptors reside then go on to encode the information about multiple odorants, in a manner that the rest of the brain can discriminate between them? Does it require a temporal-coding mechanism? Because it seems to me that's the only way a single neuron could signal so many different odorants. Using a simple firing-rate mechanism would make it's informational capacity pretty limited.
Problem there is that the temporal-coding stuff hasn't really gone anywhere; the rate vs. timing debate doesn't seem to have shifted in favor of the temporal-coding supporters. So I guess I am at a loss as to how one links this idea up with what we know about sensory processing in general. It's all fine and well to have a molecule that responds differentially to odorants (rather than just on/off) but unless its tied to a neuron (or neurons) that are capable of then encoding that information somehow, it's not going to get that information into the system.
Unless of course you want to suggest that the entire system then somehow goes on to "vibrate" in resonance with the odorants, and then of course you're off into la-la land.
Could you please discuss what is known about the higher-level olfactory processing as well? It seems to me that if indeed the nose is a sort of spectrograph, then the olfactory processing should resemble auditory processing, where different ear cells respond to different sound frequencies.
Your suprisingly positive account of Turin's theory of quantum olfaction ignores one of very few experimental tests of predictions of the theory - A psychophysical test of the vibration theory of olfaction by Andreas Keller & Leslie B Vosshall, published in Nature Neuroscience 7, 337 - 338 (2004). Briefly, the tested 3 of Turin's predictions. One compelling test the authors performed was evaluating the ability of human subjects to discriminate between acetophenone and completely deuterated acetophenone. These two molecules have the same shape, but different molecular vibrations. Thus, the lock and key hypothesis and the tunneling hypothesis generate conflicting predictions - the former predicts these molecules shouldn't be discriminable, while the latter says they should. Using a variety of psychophysical methods, Keller and Vosshall show that the two molecules are indistinguishable as far as your olfactory epithelium is concerned. Other tests of Turin's predictions turn up similar results.
The popular press (particularly the book Emperor of Scent, a rather feverish account of Turin's work) and Turin himself have implied that there is some kind of conspiracy in the scientific establishment to suppress his work. No such thing - his ideas failed to generate much interest because he seemed remarkably disinterested in generating doing experiments to test his hypothesis. To this day his scientific publications on the tunneling hypothesis are confined to reviews and theory papers.
Initially intrigued by Turin's model, I'm now a little worried. Is the synopsis given here indeed correct? It would seem to imply that a given receptor can�by itself�discriminate odors. That would not be in keeping with studies that imply that it is the odorant receptor neuron, and not the receptor itself, that determines odor perception.
Yes, my synopsis of the model is correct. Turin says so himself in a comment on the original post.