In his post last Friday, Peter did a very nice job of introducing the the counter-intuitive idea that having too little fat, rather than too much, causes many of the metabolic problems of obesity. Today I thought it would be good to continue on with this theme and to focus on some of the mechanisms that explain this strange relationship. Let's begin where Peter ended off:
Currently, the emerging theory of why obesity is associated with metabolic disease risk suggests that it is not the excess amount of fat that results in problems - but rather, it is the inability of the fat tissue (specifically subcutaneous) to expand enough via the development of numerous, healthy adipocytes or fat cells to store all the excess calories being ingested.
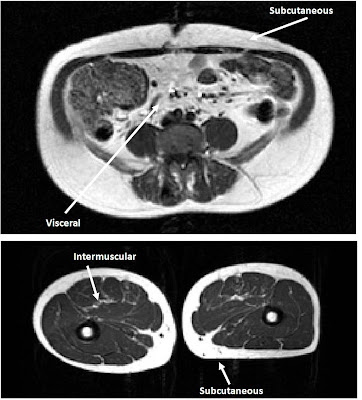
Fat tissue is made up of many small fat cells, called adipocytes, each of which stores a single droplet of lipid. When we say that there are problems with having too little fat, we really mean that there are problems with having too few adipocytes. For the purposes of this discussion, the most important categories of adipocytes are subcutaneous (those found just below the skin), visceral (those found inside of the abdominal wall) and intermuscular (between muscles). I've taken the figure below from my Master's thesis, which shows where each of these types of cells can be found within the body.
What's interesting about these different types of adipocytes is that they differ in their rates of lipolysis - essentially how rapidly they release fat into the blood stream. In comparison to subcutaneous adipocytes, visceral adipocytes have higher rates of lipolysis and decreased sensitivity to the anti-lipolytic effects of insulin. What this means is that visceral adipocytes are very quick to give up the fat they store, while subcutaneous adipocytes (especially those found in the lower body) hold onto fat, and really resist letting it into the bloodstream. When visceral adipocytes give up their fat, it circulates through the bloodstream where it can accumulate within the liver, heart, and skeletal muscle, resulting in insulin resistance and metabolic dysfunction. In contrast, subscutaneous adipocytes hold tightly onto their fat, preventing it from doing any damage to the rest of the body (click here for a fantastic in-depth review of the differences between visceral and subcutaneous adipose tissue by Bernardo Wajchenberg).
Let's say that you're a lean, healthy individual, but for one reason or another you begin to consume more calories than you burn. You are going to start storing fat in your subcutaneous adipocytes. As Peter suggested, so long as you are able to continue storing fat in those subcutaneous adipocytes, you are likely to be metabolically healthy. However, if you continue to gain weight, you will eventually exceed the storage capacity of your subcutaneous adipocytes, at which point fat will start to "overflow" into visceral adipocytes. As mentioned earlier, these visceral adipocytes already have high rates of lipolysis, which is bad enough. But as they enlarge, visceral adipocutes become increasingly insulin resistant, resulting in the release of even higher amounts of fat into the bloodstream, rapid accumulation of fat within the liver and other organs, and increased metabolic risk.
The simple differences in visceral and subcutaneous adipocytes can help to make sense of the "outliers" that Peter discussed in his post on Friday. Individuals with lipodystrophy (who have very few adipocytes) appear lean, but with no place to safely store their fat, it rapidly accumulates within the heart, liver, and muscles, resulting in increased health risk. Similarly, TZD treatment results in an increase in the number of subcutaneous adipocytes, which essentially suck up circulating lipid and reduce metabolic risk accordingly. This is also why it can be so difficult to reduce the amount of fat stored in your hips and thighs - those subcutaneous adipocytes just do not want to release the fat that they are storing! It may be frustrating, but it's terrific from a health perspective!
So, what is the clinical utility of these differences? Unfortunately, not much. We really have no control over where we store our body fat. And the simple fact is that most obese individuals have large amounts of visceral fat, resulting in increased health risk. But I still find it incredibly interesting that, far from being the scourge that people often expect, fat cells are actually extremely important to the healthy function of the human body.
Travis
- Log in to post comments


Nice follow up to the earlier post!
I know you guys did your best to write the last two posts in plain language for the non-adipose nerds in your readership, but I still don't get it. Does this mean that all skinny bastards like me are considered at risk of early mortality? Or just lazy, skinny bastards like me? How is this related to exercise? Hey, you started it ...
If you don't explain this further, I'm cancelling my subscription to your blog, and going back to reading Douchebags with Hot Chicks; it's much easier to understand.
Thank you for the post!
After following your blog, I do see one utility of this. People with metabolic syndrome should do more resistance training. This could help reduce visceral fat and result in better health outcomes than just focusing on weight loss with unhealthy methods.
It is better to have healthy diet and exercise.But, if someone cannot manage everything, then it is atleast useful to direct them to focus on fat loss.
clear explanation of the types of fat cells. Thanks.
Wow, I can't believe that that is actually a website. Almost as good as Awkward Family Photos.
As to your question, even though you are lean, you would still have plenty of adipocytes (assuming you don't have lipodystrophy, of course). If you have very little body fat then most of your adipocytes (both visceral and subcutaneous) will be in peak condition - small, and insulin sensitive. The more body fat that you have, the greater the likelihood that you have begun storing visceral fat. But as I mentioned in the post, exercise preferentially reduces visceral fat, and it has been suggested that it might be better to be overweight and physically active, rather than lean and inactive. So having a healthy body weight is a good thing, but being physically active is just as important, if not more so.
Travis
This is a joke, right? Have you even taken an endocrinology course? Know anything about NAFLD? It isn't caused by "fat spilling" into the blood from fat cells. To say this is what causes insulin resistance anywhere or, more specifically, the liver, is not true. Have you ever seen anyone that was otherwise lean but looked like they had a basketball in their stomach? Ever tried to measure skinfolds on someone like this? It's all visceral fat and it didn't get there from subcutaneous fat "spilling over" and preferentially ending up as visceral fat.
The differences between men and women and fat storage are hormonal, mainly testosterone and estrogen. Go back and read section VI. Here is the last sentence from the first paragraph in sub-heading "B":
"Available information does not indicate that visceral adipose tissue contributes much to liver exposure of FFA"
The quote goes completely against what you stated about subcutaneous and visceral fat. Furthermore, the main cause of NAFLD is dietary fructose, primarily the overconsumption of the pervasive and ubiquitous ingredient HFCS. All of which has absolutely nothing to do with any type of fat cell "spilling over" into the blood stream and targeting the liver.
While we may not have much control over where our body stores fat, we certainly have control over how much it stores. A problem, for the most part, which can be regulated by the foods we eat.
Jordan
Hi Jordan,
I'm going to have to disagree with you, and not just because you jumped immediately to ad hominem attacks. Of course fat is not literally spilling out of adipocytes, but it is a useful way to conceptualize what is happening, and the "lipid overflow model" is an accepted term in this area of research. For another terrific review from Nature which walks through the overflow model in detail (including its likely contribution to hepatic insulin resistance) click here. Or consider this sentence from a 2004 review in the Journal of Clinical Endocrinology and Metabolism:
"Enlarged fat cells are insulin resistant and have diminished capacity to store fat. When adipocyte storage capacity is exceeded, lipid âoverflowsâ into muscle, liver, and perhaps beta-cells, causing muscle/ hepatic insulin resistance and impaired insulin secretion"
So I can understand that you might disagree with my arguments, but you have to be willing to completely ignore a large body of evidence to summarize this entire area of research as simply "not true".
The line that you quote from Wajchenberg is (I believe) referring to the fact that it is unclear whether the majority of the fatty acids that make their way to the liver originate in visceral adipocytes, or abdominal subcutaneous adipocytes. As they point out in that same paragraph, the rate of lipolysis is higher in visceral adipocytes, but visceral fat often makes up a much smaller absolute volume than abdominal subcutaneous adipose tissue. The result is that it is very possible that abdominal subcutaneous adipocytes (which act more like visceral adipocytes than subcutaneous adipocytes found in the lower body) contribute a greater proportion of the total fatty acids which reach the liver - an issue which is discussed nicely by Klein et al. There is also some evidence suggesting that the accumulation of visceral fat causes an increase in lipolysis in abdominal subcutaneous adipocytes, indirectly influencing the amount of lipid that eventually reaches the liver. Suffice it to say that it's a complicated issue, but the Wajchenberg review is hardly a rebuttal of our central arguments - in fact the review itself clearly argues that visceral lipolysis plays an important role in the development of insulin resistance in Figure 3.
The idea that someone could have a basketball stomach but be otherwise relatively lean fits perfectly within the idea of the lipid overflow hypothesis - the individual has very little ability to store fat subcutaneously, therefore the fatty acids accumulate in other depots, including visceral adipocytes. This is similar to the lipodystrophic condition that Peter discussed in his post last week. And I won't disagree that sugars in general (and probably HFCS in particular) are also a major contributor to metabolic dysfunction. There is strong evidence for that as well, but there can certainly be more than one contributing factor to NAFLD.
I'd welcome a genuine discussion of these issues, so if you can provide links to data that you feel dispute my arguments then please add them to a comment below. But if you simply want to compare endocrinology course credits, you're probably in the wrong place.
Travis
There is some metabolic truth in your statements. The regulation (or dysregulation) of fat tissue is the same, whether it's subcutaneous or visceral. It's controlled by lpl, which is regulated by several hormones, but most strongly by insulin.
True, visceral fat is more dangerous than subcutaneous fat, but the implication is someone with little visceral fat and 100 pounds of subcutaneous fat is better off than someone with little or no visceral fat and 30 pounds or so of subcutaneous fat is not being scientifically correct. This is the implication in your post. The 100 pounds of subcutaneous fat is a metabolic problem, not very protective.
The key to all of this, of course, is insulin resistance. Fat cells, regardless of whether visceral or subcutaneous are becoming insulin resistant long before they are actually labelled as resistant, readily identified by slow but steady weight gain starting for most in their 30's. This appears to be independent of physical activity, too.
For a type 2 diabetic, insulin resistance is the salient point, not whether the fat goes to subcutaneous or visceral fat stores. Insulin resistance is the salient point for too much fat, regardless of diabetes diagnosis. Elevated insulin makes it very difficult, if not impossible, to release fat from the fat tissue. It can store it but releasing it is a problem.
So you get this cycle of storing fat but not using it, hyperinsulinemia. Fixing this, which can be done through dietary changes - as long as the pancreas is still functional, fixes fat dysregulation, independent of subcutaneous or visceral.
The first step in dietary changes is to remove all forms of sugar (particularly HFCS), most fruit (too much fructose which has to go through the liver) and other high GI carbohydrates. This does two things: significantly reduces impact on the liver and drops triglycerides. It also reduces circulating levels of insulin, allowing fat to be released from the fat cells and used for energy, as opposed to their hyperinsulinemic state of storage.
As far as references go, the regulation of fat tissue has been known for many years. Any medical physiology text should have it clearly detailed. For more recent info, just go to pubmed and search hyperinsulinemia and adipocyte.
Jordan
Hi Jordan,
Thanks for elaborating. Bob Lustig has made similar arguments about sugar and especially fructose causing increased metabolic risk. While those arguments are pretty persuasive, they don't refute the evidence that visceral fat is also a major contributor to increased metabolic risk. They are simply separate risk factors, in the same way that inflammatory proteins (which we have ignored so far in this discussion) are also a very likely cause of metabolic dysfunction. The presence of one mechanism doesn't disqualify the others. If you know of specific studies that cast some doubt on the mechanisms that I mentioned in the post (rather than simply discussing other separate mechanisms) then it would be great to discuss them in more detail. But if you're not willing/able to share the references that refute these mechanisms, it's tough to get to the bottom of what's actually going on. I'm not being disingenuous - I think the spillover hypothesis makes a lot of sense (not to mention having a solid body of evidence behind it), but if there's a study that disproves it or even suggests that it needs to be re-considered, I'd really like to read it.
I think you're overstating things when you say that "elevated insulin makes it very difficult, if not impossible, to release fat from the fat tissue" - all of the papers that I referenced in my earlier comment have suggested that lipolysis continues once insulin resistance is present (a situation where hyperinsulinemia is also likely), and probably even intensifies from visceral adipocytes. Or consider this finding from a study published in Diabetes:
"Our data indicate, however, that chronic hyperinsulinemia may induce increased rates of lipolysis in contrast to its acute effects. This is the first report to address the long-term effects of insulin on human adipocyte lipid metabolism, and our findings are consistent with clinical observations of the association of hyperinsulinemia with increased lipolytic rate ..."
Again, providing some references to back-up your arguments could make me reconsider my viewpoint.
I'm not sure that my post was suggesting that someone with 2 lbs of visceral fat and 2 lbs of subcutaneous fat is any worse off than an individual with 2 lbs of visceral fat and 30 lbs of subcutaneous fat, although previous cross sectional studies by both Peter and myself have suggested that that may actually be the case. It's certainly counter-intuitive, but in what way is it not "scientifically correct"?
Travis
Thank you both for this stimulating and informative discussion.Us curious lay-people are much better equipped to understand "why we are unrelenting in maintaining our weight losses w/ stringent avoidance of "sugar" products and high glycemic "flour" products, or "bad carbohydrates" through programs such as Food Addicts in Recovery Anonymous. We may not understand all the metabolic significance of our abstinences, however, we've seen the often massive weight loss results and the accompanying reduction in medications for pre-diabetes, high blood pressure and high cholesterol. Getting the gist of this discussion gives me additional ammunition to pursue further careful eating and exercise habits to hopefully reduce the "womanly" (?) subcutaneous fat in the hip/rear region. There IS STILL hope! I'm "only" 54 w/ years of sveltness ahead! And hopefully (again) avoidance, or at least delay of the heart disease plaguing both my too-young parents, and diabetes and other bizarre auto-immune issues my mother is combatting. I'll cross my fingers for extra luck also, that it isn't too late for me from the "very-fat-decade" post-pregnancies after a life-time of "skinniness" but very poor eating habits.